Epilepsy: animal experiments and animal-free research
Epilepsy is a neurological disorder that affects approximately 50 million people worldwide. In Germany, around 800,000 people receive medical treatment for epilepsy (1). However, current treatment options remain unsatisfactory, with up to one-third of patients not benefiting from available medications. Further research is needed to develop more effective treatments and improve patients' quality of life. But how is this research conducted, and is it both ethically justifiable and scientifically meaningful?
Epilepsy is characterized by recurrent seizures caused by sudden, excessive electrical activity in the brain. These seizures can vary widely, ranging from brief moments of absence to severe convulsions. A distinction is made between generalized and focal seizures. In generalized seizures, the entire brain is affected, often leading to unconsciousness and full-body convulsions. Focal seizures originate in a specific area of the brain. Depending on the function of the affected region, symptoms may include twitching of individual limbs, sensory disturbances, or changes in vision. Hallucinations and anxiety attacks are also possible (2).
Available treatment options
The treatment of epilepsy primarily includes medication, surgical interventions, and non-pharmacological approaches such as neurostimulation.
- Medication: Antiepileptic drugs are the first-line treatment for epilepsy. They work by reducing neuronal excitability or modulating signal transmission in the brain. While many patients respond well to these medications, about one-third remain drug-resistant (3).
- Surgical Interventions: If seizures are localized to a specific brain region, surgical removal of that area can significantly reduce or even eliminate seizures (4).
- Non-Pharmacological Approaches: These include the ketogenic diet (5) and neurostimulation technologies such as vagus nerve stimulation or deep brain stimulation (6).
Despite these diverse treatment options, epilepsy remains difficult to manage for many patients. Medication is ineffective for approximately 30% of patients (2), and some drugs have severe side effects. As a result, research continues to investigate the condition and new treatment methods, with many researchers still using animal experiments.
Animal experiments in epilepsy research
Animal experimentation has been a part of epilepsy research for decades. Rodents, particularly mice and rats, are primarily used to understand the mechanisms of the disease and to test new therapies. Common "models" include genetically modified animals or those in which epilepsy-like conditions are induced through chemical substances or electrical stimuli.
Chemical induction
In chemical induction, various substances are injected into animals either systemically or directly into the brain.
One example is the compound pilocarpine, which triggers hyperexcitation of certain synapses, leading to epileptic conditions. It is often injected into the abdominal cavity (7) and causes a mortality rate of 25 to 100% in mice, with many animals dying from respiratory failure due to seizures (8). To reduce this high lethality rate, atropine can be administered before the pilocarpine injection. After receiving pilocarpine, the animals experience seizures, which can be fatal in 10 to 30% of cases. If a generalized seizure lasts longer than 30 minutes, the sedative diazepam is administered. Spontaneous seizures begin to occur about 1 to 4 weeks after induction.
Another compound, kainic acid, induces excessive neuronal excitation, particularly in the hippocampus. Like pilocarpine, kainic acid is injected into the abdominal cavity but can also be directly administered into the brain. This leads to epileptic seizures and can cause neuronal cell death (9).
Tetanus toxin is also used to induce epilepsy-like conditions in animals. It is injected directly into the brain. For example, rats are anesthetized, and their heads are fixed in a stereotactic frame. A hole is drilled into the skull, through which a needle is inserted into the brain. The tetanus toxin is then injected in a small volume of liquid. Recurring seizures typically begin 1 to 3 days after injection (10). In some cases, electrodes are implanted into the animals' brains to monitor and measure seizure activity. Researchers then study how different compounds affect the seizures (10).

Mice are injected with chemicals that induce epilepsy-like seizures.
Electrical stimulation
In this approach, electrodes are implanted into the animals' brains to deliver targeted electrical stimuli. Mice or rats are anesthetized, their heads are secured in a stereotactic frame, and a hole is drilled into the skull. Electrodes are inserted through this hole into the brain and fixed to the skull. After a recovery period of several days, electrical currents are applied to the electrodes, inducing epileptic seizures in the animals.
The so-called "kindling model" induces an epilepsy-like state through repeated, mild electrical stimulation of specific brain regions. Initially, each stimulus does not trigger a seizure. However, over time, these repeated stimulations cause changes in the brain, making it increasingly sensitive to stimuli, eventually leading to more severe and prolonged seizures. Researchers believe that this process mimics the development of epilepsy, so the kindling model is used to study the mechanisms underlying epilepsy. It is also employed to test the effectiveness of antiepileptic drugs and other therapies (11).
Genetic models
In these "models," animals are bred or genetically modified to spontaneously develop epilepsy-like conditions.
A common approach involves genetic modification of ion channels, which play a crucial role in neuronal excitability. For example, in Scn1a-knockout (KO) mice, the sodium channel NaV1.1 is genetically deactivated. This disruption affects inhibitory neurons (interneurons), leading to hyperexcitability and a lower seizure threshold. The imbalance between excitatory and inhibitory signals in the brain results in severe seizures (11).
Additionally, mice are genetically modified to carry specific mutations associated with epilepsy in humans. For instance, mutations in the PTEN gene, which in humans are linked to tuberous sclerosis, cause abnormal cell growth and lead to brain malformations. These alterations disrupt neuronal circuits and trigger epilepsy-like conditions (11).
Beyond these examples, many other genetically modified animal models exist. Most of these are genetically engineered mice, as genetic manipulation techniques are well-developed and widely available for this species. However, genetically modified zebrafish are also used in epilepsy research (11).
Other models
In gerbils, epilepsy-like conditions can be induced by exposing these noise-sensitive animals to intense sounds (11). Baboons are also used in epilepsy research (12). In these animals, epilepsy-like seizures can be triggered by strobe light (flickering light) stimulation. During stimulation, generalized seizures can occur, lasting 20 to 30 seconds (11).
In all so-called epilepsy models, the suffering of the animals is immense. In addition to acute seizures, many animals experience chronic neurological and physical issues caused by the induced conditions.
The failure of animal-based epilepsy research
The success rate of animal-based drug development — the likelihood that a substance that has been tested as effective and safe in animal studies will also work in human clinical trials and receive approval - is, on average, less than 7% across all indications (13).
Medications developed in the field of neurology, which includes epilepsy, have a particularly low success rate, with only 5.3% of drug developments succeeding (13). This means that nearly 95% of substances aimed at treating neurological diseases, despite seemingly positive results in animal studies, fail in human clinical trials. Most of these substances fail in Phase II of clinical trials (14), which is when their effectiveness is first tested on patients with the disease. This demonstrates that preclinical research, which heavily relies on animal experiments, fails due to the lack of translatability to humans.
Below, the reasons for the poor translatability of animal experiments to humans in the field of epilepsy research are briefly summarized.
Differences between species
The physiology and brains of animals differ significantly from those of humans. As a result, many findings from animal studies cannot be translated to humans. Medications that are effective in animals often show no effect or unexpected side effects in humans.
The brains of rodents, which are most commonly used in epilepsy research, differ greatly from the human brain in terms of size, structure, and neural organization. For example, the hippocampus in mice is larger in relation to total brain mass, while the human brain has a more developed cortical complexity. In humans, the neocortex plays a central role in many forms of epilepsy, whereas in rodents, epilepsy is often "modeled" (i.e., artificially induced) in the hippocampus. Thus, the environment in which epilepsy is studied in animals does not correspond to the situation in the brains of humans with the disease.
In genetic animal models, individual genes are manipulated to simulate genetic forms of epilepsy. These models best replicate monogenic epilepsies, which are based on changes in a single gene. However, monogenic epilepsies are rare in humans (15). In contrast, epilepsies in humans are often polygenic or multifactorial, which cannot be represented by animal studies.
Artificial disease models
The epilepsy-like conditions induced in animals differ fundamentally from the human disease. For example, they often lack the complexity and diversity that characterize human epilepsy. Epilepsy is artificially induced in animal models, such as through chemical (pilocarpine, kainic acid) or electrical stimulation or through genetic manipulation. These methods simulate certain aspects of epilepsy (e.g., focal or generalized seizures), but they do not reflect the multifactorial nature of human epilepsy.
Epileptic seizures in animals often follow a uniform pattern, determined by the inducing methods (e.g., chemical induction). These seizures can differ in duration and intensity from human seizures. In contrast, humans experience a wider range of seizure types, which can vary greatly in length and intensity. Additionally, emotional factors and external stimuli influence seizure dynamics in humans, which cannot be adequately accounted for in animal studies.
Chemical and electrical inductions often lead to an acute seizure status, followed by a phase of chronic epilepsy. After weeks or months, spontaneous seizures occur, but typically in predictable patterns. In humans, however, seizures can occur spontaneously, often without known triggers. The chronic course and unpredictability of seizures, as well as the wide range of symptoms, make human epilepsy much more complex.
In addition to the scientific reasons behind the failure of animal-based epilepsy research, there are further arguments against animal experiments. In epilepsy research, animals are deliberately subjected to significant suffering. Moreover, animal experimentation is costly and time-consuming, while the success rate of translating the results to humans remains disappointingly low. This raises the fundamental question of whether animal experimentation not only fails to promote progress in epilepsy research but may even hinder it and thus be at least partly responsible for the lack of significant breakthroughs.
As a result, there is growing effort to develop animal-free methods for epilepsy research.
Animal-free research models
Modern cell cultures, particularly three-dimensional brain organoids, allow for the study of human brain tissue in vitro. These models can also capture the genetic and biochemical characteristics of individual epilepsy patients.
Cell culture
In the simplest case, neuron cell cultures are used. Until now, these cells often came from animals killed for this purpose, as human neurons were difficult to obtain for ethical reasons. However, thanks to human induced pluripotent stem cells (iPSCs), it is now possible to produce human neurons. For example, skin cells from humans can be used and "reprogrammed" into iPSCs. From these iPSCs, various cell types can be generated through directed differentiation, including neurons. The advantage of this method is that research can be conducted directly on human cells, which can also be obtained from individual patients. This makes neurons derived from iPSCs especially suitable for studying genetically determined forms of epilepsy and offers the possibility to personalize therapies for individual patients (16).
However, simple cell culture systems lack the structural complexity of the brain, where various cell types interact with one another. Therefore, three-dimensional cell cultures, such as organoids, are increasingly being used in epilepsy research.
Brain organoids
Brain organoids are three-dimensional structures that can be derived from iPSCs. They can replicate the structure and cellular diversity of the human brain. To create them, iPSCs are converted into neural progenitor cells, which then organize themselves into complex structures. These structures resemble various brain regions, and the process of their formation mimics the development of the human brain in the embryo (17).
These brain organoids can form neural networks and exhibit functional properties of real brain tissue. Brain organoids are particularly useful for studying the cellular and molecular mechanisms behind epilepsy. They allow scientists to investigate how certain genetic mutations affect the development, connectivity, and excitability of neurons. Additionally, organoids can be used to test the effects of potential medications on seizure activity (17).
Electrophysiological measurements can also be performed on brain organoids (17). For instance, Wu et al. (2022) developed brain organoids from stem cells of two patients with a neurological developmental disorder caused by mutations in the CDKL5 gene (CDD). Affected patients exhibit developmental delays, early-onset seizures, and autistic behavior, which are linked to the overexcitability of neurons. Using special electrophysiological techniques, Wu et al. analyzed how nerve cells in these brain organoids transmit signals and generate electrical impulses, comparing them to healthy control samples. The organoids exhibited similar overexcitability to that seen in the patients, demonstrating that the disruption of ion channels plays a central role in the disease's development (18).
In addition to studying disease mechanisms, brain organoids can also be used in drug development. For example, Yokoi et al. (2021) used a special method by combining brain organoids with a multi-electrode array. Using this approach, they studied the response of organoids to seizure-inducing substances and various medications with different mechanisms of action. This method proved useful for both, determining the undesirable seizure potential of medications, and assessing the activity changes of the organoids after the administration of antiepileptic drugs (19).
Thus, brain organoids represent a modern, human-oriented tool in epilepsy research, providing deep insights into human-specific disease mechanisms that cannot be captured by animal models.
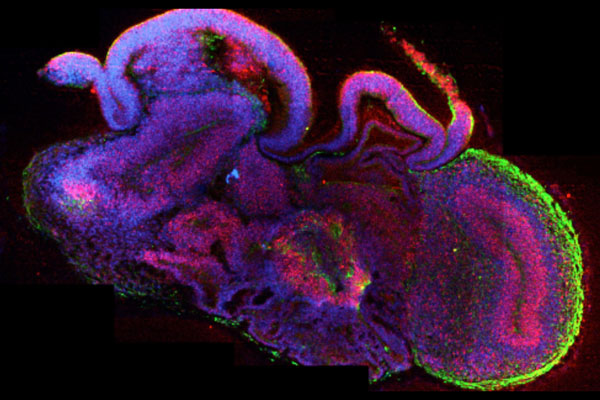
Brain organoids replicate the structures of the human brain and exhibit functional properties.
©Madeline Lancaster (Lancaster, M.A. Nature 2013; 501: 373-379)
Organ-on-a-chip systems
A microphysiological system (MPS) is an artificially created model made from two- or three-dimensional cell structures, referred to as organ-on-a-chip. These "chips" contain living cells that are cultivated under controlled conditions with continuous fluid flow. This setup allows for the replication of the physiological and disease-related functions of human organs.
For example, Pelkonen et al. (2020) developed an epilepsy-on-a-chip system. On the chip, three separate neuronal networks are connected via fine microfluidic channels. The activity of these networks can be monitored with specialized microelectrodes. The networks exhibited spontaneous activity patterns that were synchronized both, locally and between the interconnected networks, similar to brain functions. When the seizure-inducing substance kainic acid was added, the activity was only intensified in the treated networks. The antiepileptic drug Phenytoin successfully reduced the activity of the overexcited networks (20). The developed system enables, for the first time, the modeling of focal seizures in human neuronal networks and provides a new platform for studying epilepsy and potential therapies.
This innovative technology could significantly improve and streamline drug development, potentially replacing animal experimentation in preclinical research (21).
Computer-assisted models and AI
The analysis of large datasets generated by the study of complex tissues and organs requires modern technologies such as artificial intelligence (AI) and machine learning (ML). AI-based algorithms can analyze continuous data, such as EEG recordings, to predict the risk of seizures. This enables patients and doctors to take preventive measures. Additionally, AI/ML models can predict how a patient will respond to specific antiepileptic drugs based on patient data such as genetic information, therapy history, and clinical features (21).
Finally, AI can also be used to analyze large datasets, such as those generated in organ-on-a-chip experiments. The combination of modern in vitro methods with AI/ML could thereby contribute to the development of new therapies (21).
While the results from animal experiments cannot be transferred to humans due to species differences and the discrepancies between artificially induced epilepsy-like states and actual epilepsy in human patients, animal-free methods allow for investigations directly on humans or human material. Moreover, they not only provide results relevant to humans but also enable personalized therapies tailored to individual patients.
Etiology
The exact pathways leading to epilepsy are not yet fully understood, likely due to the use of inappropriate model systems in research (17). Probable causes of epilepsy include genetic factors, brain injuries, brain tumors, strokes, infections, and lack of oxygen supply in newborns. Animal-free methods such as genome-wide association studies and the analysis of patient databases have contributed to identifying genetic risk factors.
Prevention of epilepsy, in line with the causes of the disease, primarily involves approaches to improve prenatal care, prevent head injuries, and control infections.
Beyond these primary prevention measures, which may prevent the onset of the disease but are largely beyond individual control, individuals already diagnosed with epilepsy can reduce the frequency of seizures by adjusting their lifestyle. In this context, the Epilepsy Foundation lists a number of seizure triggers that can be avoided. For example, alcohol consumption can lead to seizures, and sleep deprivation and stress can also trigger seizures. Affected patients can therefore reduce the number of seizures by maintaining a healthy lifestyle (22).
Conclusion
Epilepsy research is at a turning point. While epilepsy research was primarily conducted using animal experiments in the past, it is nowadays increasingly clear that animal experimentation is scientifically ineffective and ethically problematic. Modern, animal-free methods are not only more efficient but also better suited to capture the complexity of human epilepsy. Advances in personalized medicine, such as the use of iPSCs, allow for the creation of patient-specific models and the development of individualized therapies.
A paradigm shift toward animal-free approaches could not only prevent animal suffering but also help to develop better treatment and prevention options for millions of patients worldwide.
20.01.2025
Dr. rer. nat. Johanna Walter
Further Information
References
- Deutsche Epilepsievereinigung: Aktuelle Daten zur Epilepsie und zum Behandlungsstand
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG): Epilepsie
- Janmohamed M. et al. Pharmacoresistance – epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology 2020; 168:107790
- Sabzvari T. et al. A comprehensive review of recent trends in surgical approaches for epilepsy management. Cureus 2024; doi: 10.7759/cureus.71715
- Barañano K.W. et al. The ketogenic diet: Uses in epilepsy and other neurologic illnesses. Current Treatment Options in Neurology 2008; 10(6):410–419
- Simpson H.D. et al. Practical considerations in epilepsy neurostimulation. Epilepsia 2022; 63(10):2445–2460
- Curia G. et al. The pilocarpine model of temporal lobe epilepsy. Journal of Neuroscience Methods 2008; 172(2):143–157
- Buckmaster P.S. et al. Factors affecting outcomes of pilocarpine treatment in a mouse model of temporal lobe epilepsy. Epilepsy Research 2012; 102(3):153–159
- Rusina E. et al. The kainic acid models of temporal lobe epilepsy. eneuro 2021; 8(2):ENEURO.0337-20.2021
- Doheny H.C. et al. A comparison of the efficacy of carbamazepine and the novel anti‐epileptic drug levetiracetam in the tetanus toxin model of focal complex partial epilepsy. British Journal of Pharmacology 2002; 135(6):1425–1434
- Rubio C. et al. Classification of current experimental models of epilepsy. Brain Sciences 2024; 14(10):1024
- Szabó C.Á. et al. The baboon in epilepsy research: Revelations and challenges. Epilepsy & Behavior 2021; 121:108012
- Why are clinical development success rates falling? Biomedtracker, April 2024
- Clinical development success rates and contributing factors 2011–2020. Biomedtracker, Februar 2021
- Boßelmann C. et al. Genetische Diagnostik der Epilepsien: Empfehlung der Kommission Epilepsie und Genetik der Deutschen Gesellschaft für Epileptologie (DGfE). Clinical Epileptology 2023; 36(3):224–237
- Weng O.Y. et al. Modeling epilepsy using human induced pluripotent stem cells-derived neuronal cultures carrying mutations in ion channels and the mechanistic target of rapamycin pathway. Frontiers in Molecular Neuroscience 2022; 15:810081
- Brown R. et al. Progress and potential of brain organoids in epilepsy research. Stem Cell Research & Therapy 2024; 15(1):361
- Wu W. et al. Neuronal hyperexcitability and ion channel dysfunction in CDKL5-deficiency patient iPSC-derived cortical organoids. Neurobiology of Disease 2022; 174:105882
- Yokoi R. et al. Analysis of signal components < 500 Hz in brain organoids coupled to microelectrode arrays: A reliable test-bed for preclinical seizure liability assessment of drugs and screening of antiepileptic drugs. Biochemistry and Biophysics Reports 2021; 28:101148
- Pelkonen A. et al. A modular brain-on-a-chip for modelling epileptic seizures with functionally connected human neuronal networks. Biosensors and Bioelectronics 2020; 168:112553
- Shariff S. et al. Tailoring epilepsy treatment: personalized micro physiological systems illuminate individual drug responses. Annals of Medicine & Surgery 2024; doi: 10.1097/MS9.0000000000002078
- Epilepsy foundation: Seizure Triggers
